
Trial Master File Reference Model User Guide · PDF file16.06.2015 · Trial Master File Reference
Trial Master File Reference Model Provides an opportunity for standardization across the industry, and can be used by any company in an electronic or paper format. Framework for the Destruction of Paper 2.0. Download; Trial Master File Reference Model 3.1. Download; TMF Reference Model 3.1 Powerpoint Presentation. Download

Faqs Trial Master File Reference Model Vrogue
At the tail-end of a whirlwind year, are will psyched to percentage about thee details on and release of the Free Master File (TMF) Reference Model 3.2.0! Building on the previous update of 3.1.0 in 2018, TMF 3.2.0 earn us a host of improvements to reflect updated regulatory guidance and power industry best practices, one s fountain as ongoing feedback from the industry.
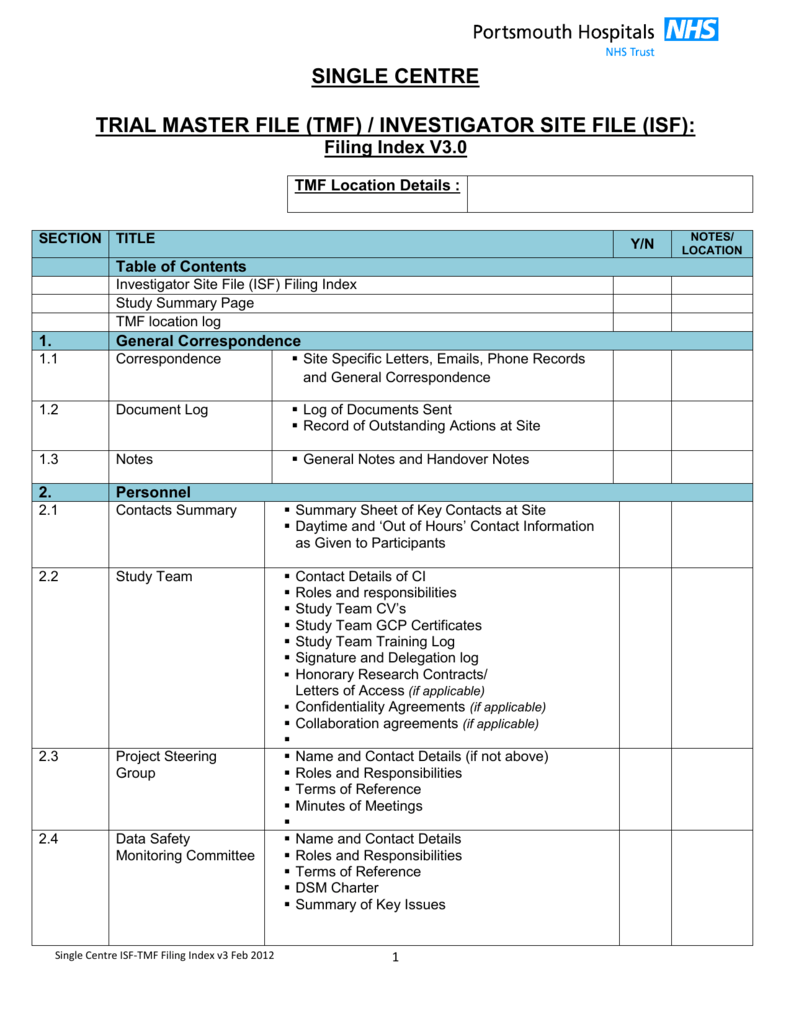
Master File Index Guide Template vrogue.co
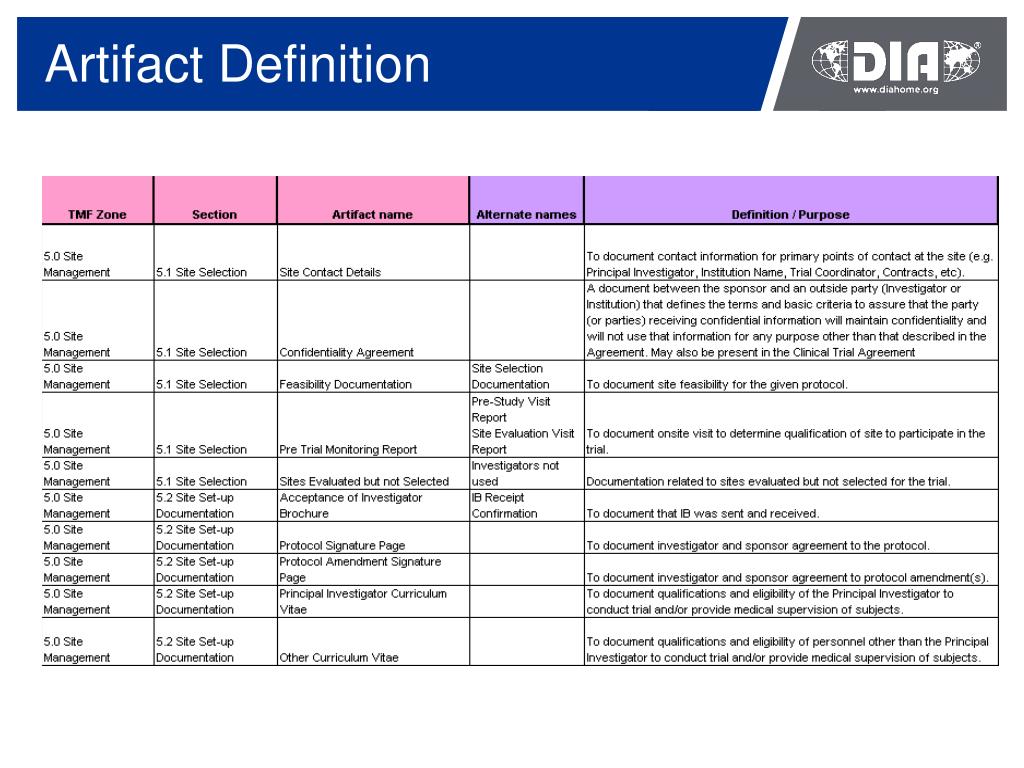
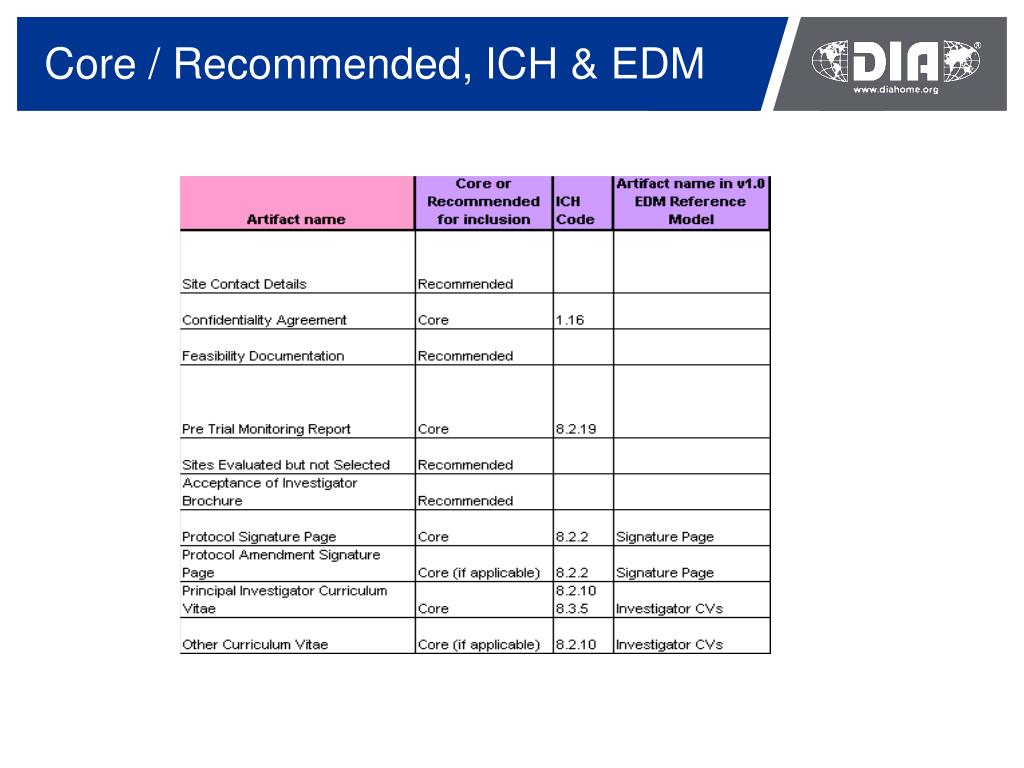
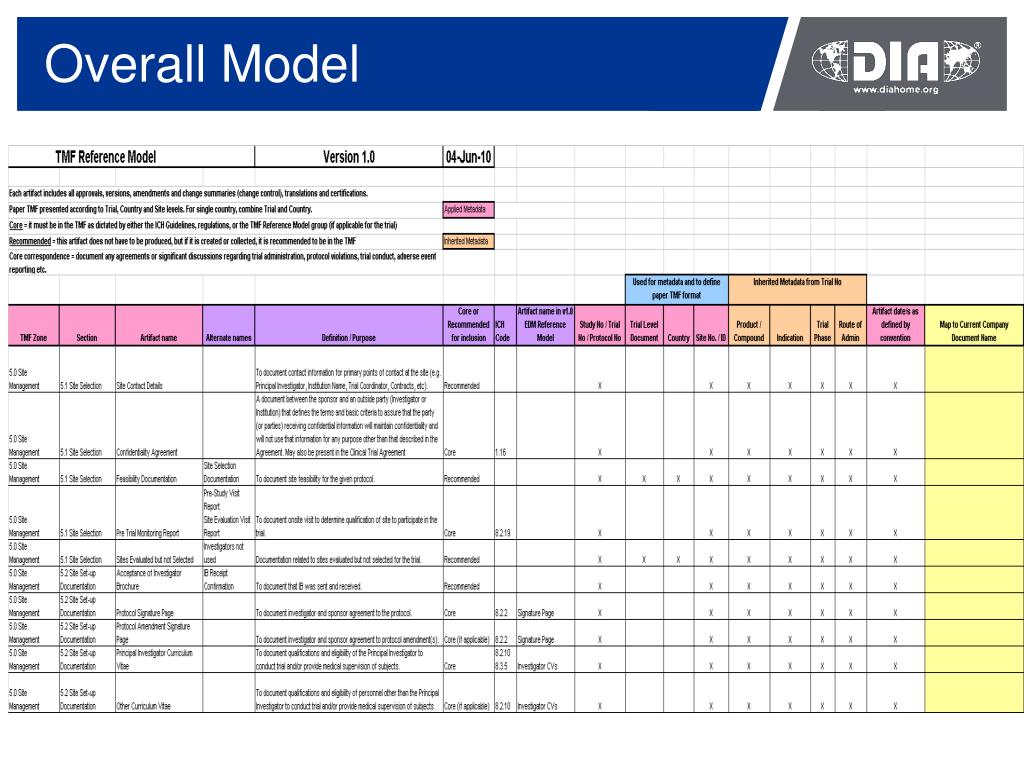
Trial Master File Reference Model. The TMF Reference Model provides standardized taxonomy and metadata and outlines a reference definition of TMF content using standard nomenclature. The TMF contains those essential documents that individually and collectively permit the evaluation of the conduct of a trial and the quality of the data produced.

TrialMasterFile Checklist [PDF Document]
Trial Master File Reference Model 3.1. This model represents the combined work of DIA volunteers in providing a model of accepted practices across the industry, and can be used by any company in an electronic or paper format. Download this TMF Reference Model to stay up-to-date with best practices in the field.
PPT DIA Trial Master File Reference Model PowerPoint Presentation, free download ID6526934
PPT DIA Trial Master File Reference Model PowerPoint Presentation, free download ID6526934
The Drug Information Association (DIA) announced the availability of version 1.0 of the Trial Master File (TMF) Reference Model. The TMF Reference Model presents a consensus position regarding the required content of the TMF, using standardized nomenclature, to include all essential documents that individually and collectively permit the evaluation of the conduct of a trial and the quality of.
PPT DIA Trial Master File Reference Model PowerPoint Presentation, free download ID6526934
The Trial Master File Reference Model (TMF RM) User Guide is a simple to use resource for introducing the TMF RM, understanding its structure, and how to use it. A separate guide describes how to implement the TMF RM. These two guides replace the original User Guide which was release in June 2015.
PPT DIA Trial Master File Reference Model PowerPoint Presentation, free download ID6526934
The Trial Master File (TMF) Reference Model is a supported initiative of the Drug Information Association's (DIA) Document and Records Management Community and represents a single, unified interpretation of the regulations and best practices related to Trial Master Files that would be accepted by all clinical trial stakeholders, with a view to be adaptable and adopted by any organization.

Trial Master File TMF Clinical Trial Systems and FDA Expectations YouTube
Building on the most widely leveraged standardized reference in TMF management today - with version 2.0 used by more than a hundred life science sponsors, CROs and technology vendors - version 3.0 of the TMF Reference Model (released at the DIA Annual Meeting in Washington, DC on June 16 th) incorporates feedback from this extensive industry use to enhance content clarity.

Presentation Slide Template (DIA) Trial Master File Reference Model
The Model is not intended to be taken and used "off-the-shelf" but can be adapted to an electronic or paper TMF, and does not endorse, nor require, any specific technology for application. DIA members and industry members are under no obligation to adopt the TMF Reference Model.
DIA Reference Model a Guidance for Good Document Management and eTMF
Trial Master File Reference Model v3.2.1;. This visual model, created by participants of a 2015 DIA conference on this topic and partially funded by an Engagement Award from the Patient-Centered Outcomes Research Institute (PCORI), depicts how we are engaging patients in benefit-risk decision making and where stakeholder efforts can improve.

PPT DIA Trial Master File Reference Model PowerPoint Presentation, free download ID6526934
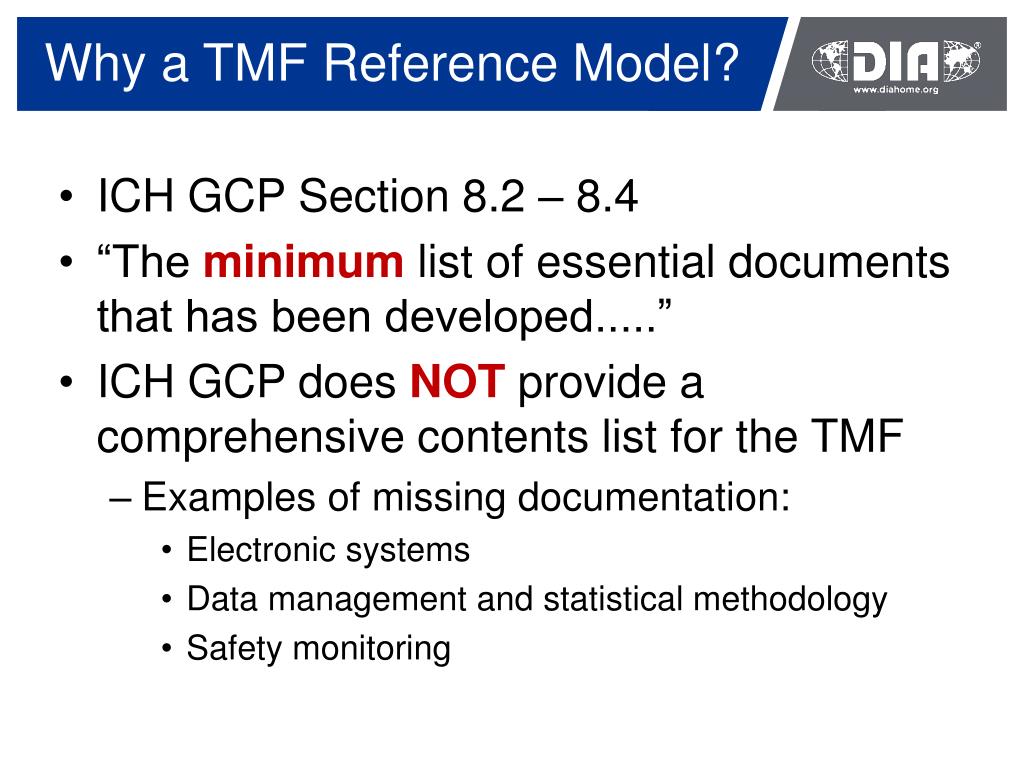
The DIA TMF Reference Model trial master file structure allows organizations to meet the "essential documents" requirements of compliance established by global regulatory bodies. These essential documents, as outlined in various regulatory guidelines (such as the FDA's regulations for the conduct of clinical trials and ICH GCP Section 8.
- Getrouwd Maar Niet Samenwonend Aow
- Kaya Artemis Resort Casino
- Hoe Laat Gaat De Mac Open
- Wat Is Er Met Vrijdag De 13e
- Jong Almere City Fc Lisse Opstellingen
- Wijk In New York 8 Letters
- Er Is Een Roos Ontsprongen Bladmuziek
- Beste All You Can Eat Overijssel
- Film George Clooney En Julia Roberts
- L Essere è E Non Puo Non Essere
