PPT GMDN Global Medical Device Nomenclature PowerPoint Presentation, free download ID653908
On this page. Global Medical Device Nomenclature (GMDN) Terms are an international naming and grouping convention used to identify and consistently describe medical devices. In Australia, GMDN Terms are a key factor in determining a 'kind' of medical device. GMDN Terms are made up of a five (5)-digit numeric Code, a Term Name and a Definition.

Table 2 from Current state of medical device nomenclature and taxonomy systems in the UK
The Global Medical Device Nomenclature (GMDN) now provides, for the first time, an international tool for identifying all medical devices, at the generic level, in a meaningful manner that can be understood by all users. Prior to the GMDN, many nomenclature systems existed, all built upon different structures, and used locally or nationally for.
UDI and the GMDN Global Medical Device Nomenclature
The nomenclature of medical devices is a coding and naming system used to generically classify and identify all medical devices and related health products. According to different classification and nomenclature systems, there are between 5,000 to 24,000 different types of medical devices. They range from the very simple to complex, inexpensive.
Common medical equipment nomenclature systems Purpose of a
nomenclatures and identifiers currently in use (UDI). Global Medical Devices Nomenclature (GMDN) was. established in 1991 and is a system of generic. description used to identif y all medical.

Global Medical Device Nomenclature GMDN PDF Free Download
The FDA supports the free Global Medical Device Nomenclature system, under development since the 1990s, that is now being used by 70 national medical device regulators from across all WHO regions.

(PDF) Global Medical Device Nomenclature (GMDN) GS1 · Global Medical Device Nomenclature (GMDN
Global Medical Device Nomenclature (GMDN) is a system of internationally agreed generic descriptors used to identify all medical device products. This nomenclature is a naming system for products which include those used for the diagnosis, prevention, monitoring, treatment or alleviation of disease or injury in humans.. The main purpose of the GMDN is to provide health authorities / regulators.

UDI and the GMDN Global Medical Device Nomenclature
Introduction. The nomenclature of medical devices is a coding system used to generically classify and identify all medical devices and related health products. Having a nomenclature system in place for medical devices facilitates their management and regulation by standardizing terms that enable communication despite linguistic and other barriers.

Global Medical Device Nomenclature Semantic Scholar
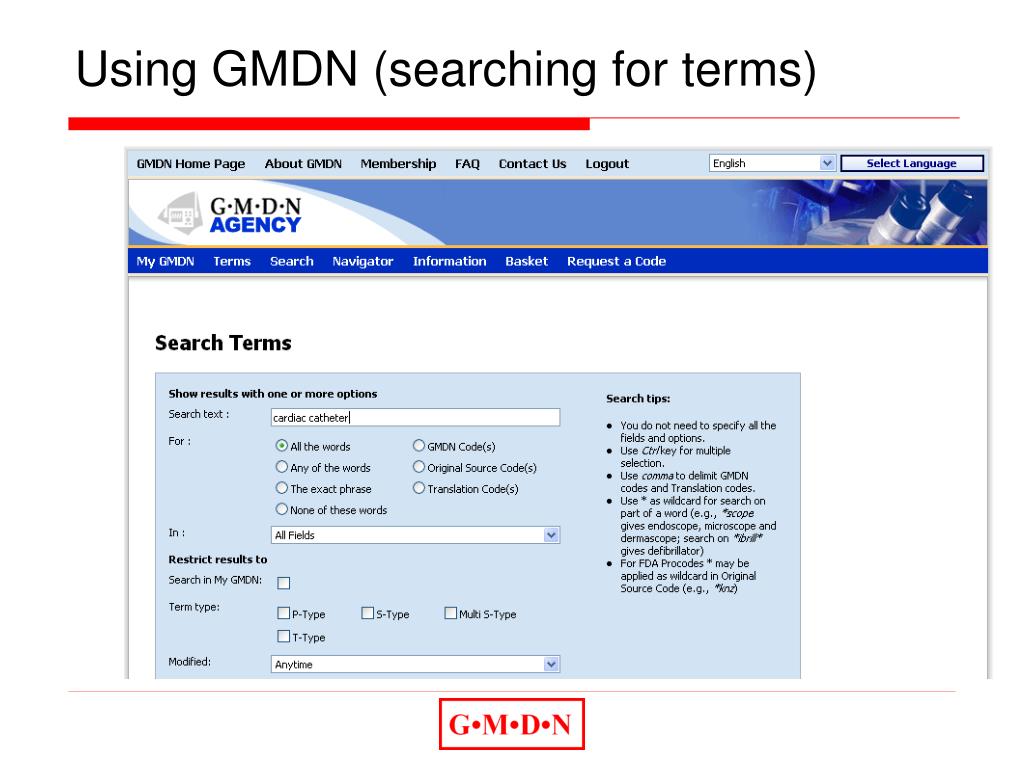
Using GMDN. All registered GMDN members can access the GMDN Database which currently has almost 25,000 GMDN Term Names which group your medical devices. Anyone can register for free as a member on the GMDN website to access and use any GMDN Term Name, Definition and Code. The GMDN has approximately 15,000 members with more than 9 out of 10 on.

(PDF) Global Medical Device Nomenclature Basic concept and FiveYear strategy
The Global Medical Device Nomenclature (GMDN) provides a classification system specifically for medical devices and diagnostics, and facilitates data exchange between manufacturers and regulators. SNOMED CT is the terminology of choice in the NHS for communicating, sharing and storing information about patients' healthcare episodes.

(PDF) PHILIPS MRI · 2019. 5
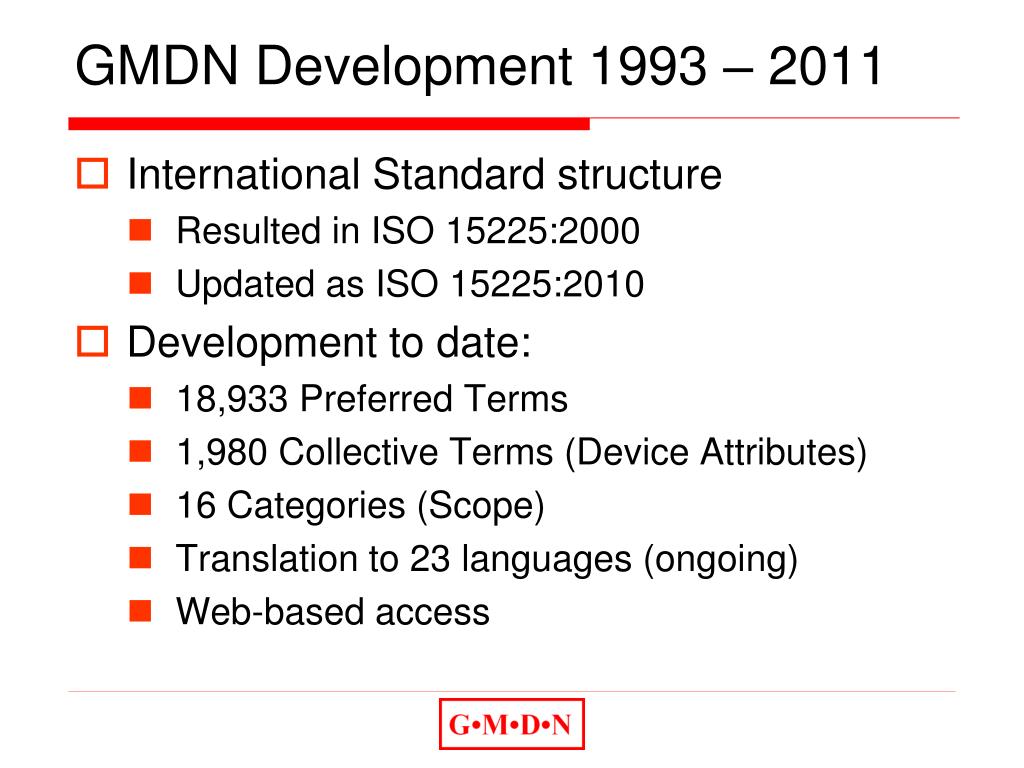
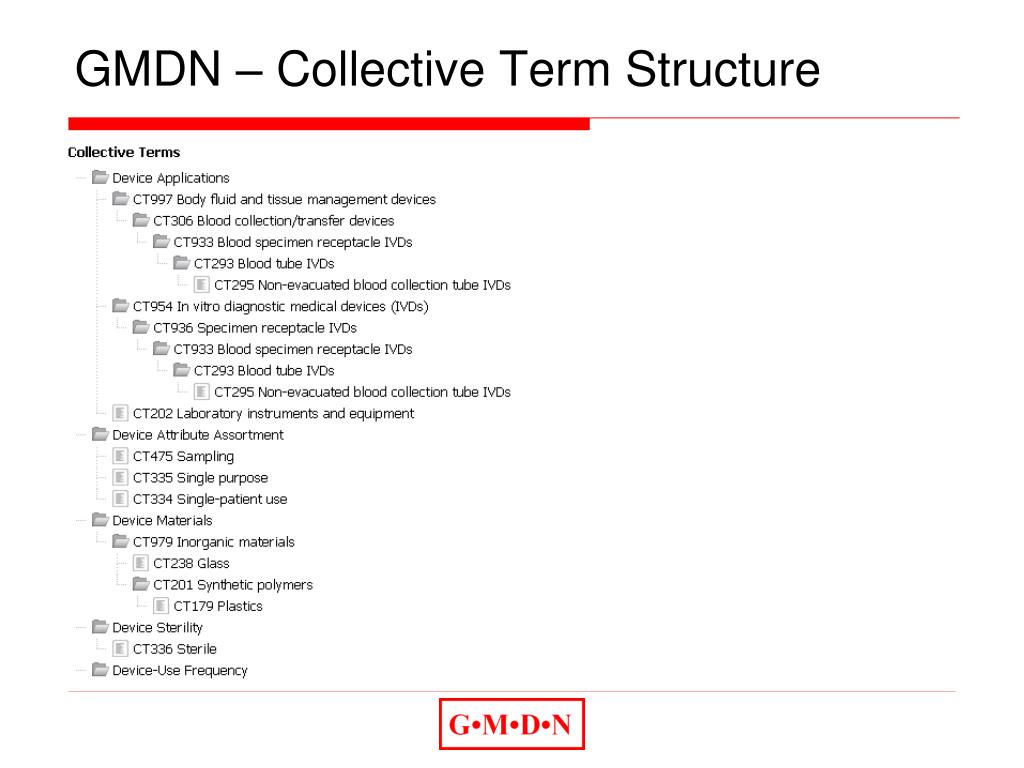
Global Medical Device Nomenclature UMDNS Issues • GMDN: International Nomenclature, provides Generic Descriptors for Medical Devices. • GMDN: based on an International Standard - ISO 15225 - which ensures that the structure of nomenclature terms are based on a consistent, standardized format.
PPT GMDN Global Medical Device Nomenclature PowerPoint Presentation, free download ID653908
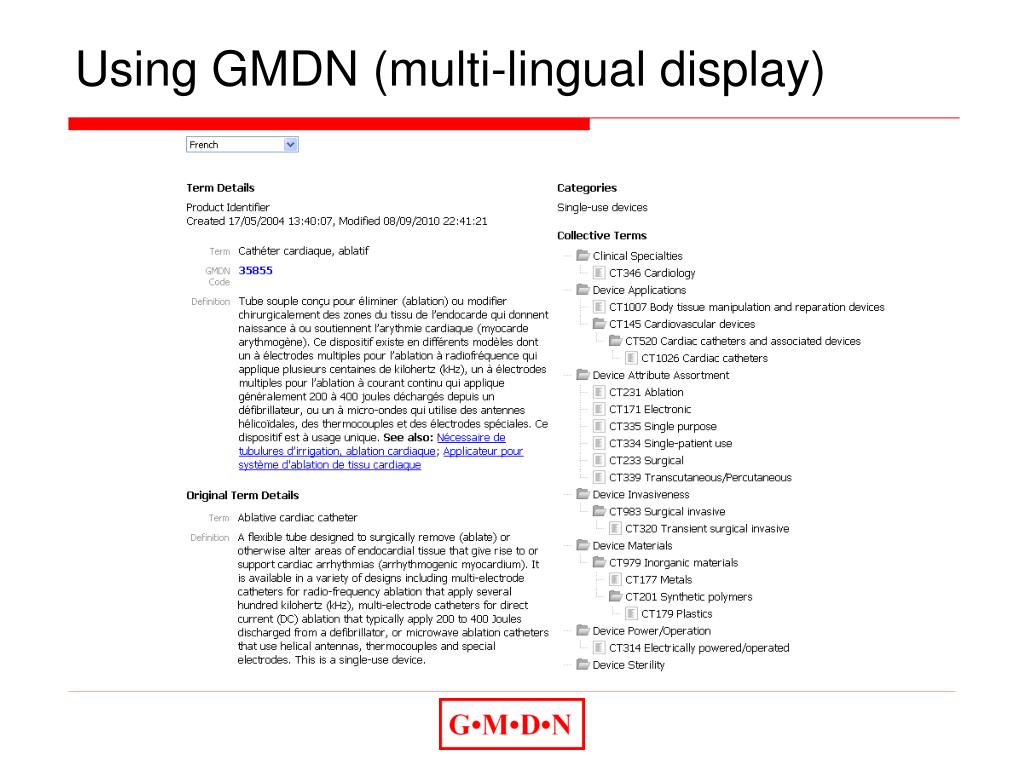
GMDN Term Structure. Each GMDN Term consists of 3 parts: Term Name General-purpose syringe, single-use. Definition "A sterile device consisting of a calibrated barrel (cylinder) with plunger intended to be used for injection/withdrawal of fluids/gas (e.g., medication) to/from a medical device or the body (i.e., capable of both).". Code 47017.

PPT GMDN Global Medical Device Nomenclature PowerPoint Presentation, free download ID653908
The Global Medical Device Nomenclature (GMDN) Code has been added to the AccessGUDID Database of medical device identification information. The addition of GMDN codes to the database creates enhanced search and retrieval capabilities for all AccessGUDID users, including patients, care givers, health care providers, hospitals, and industry..
PPT UDI and the GMDN (Global Medical Device Nomenclature) PowerPoint Presentation ID3591738
WHO data principles for medical devices: established in 2018 and published and discussed in EB145/3, EB148 and EB150. Governance: will have structures in place to ensure that all stakeholders are able to provide feedback. Classification, coding and nomenclature characteristics with: a transparent methodology and processes for updates; and.
PPT GMDN Global Medical Device Nomenclature PowerPoint Presentation, free download ID653908
The Global Medical Device Nomenclature (GMDN) provides a generic system to identify medical device products used to diagnose, monitor, treat and prevent disease or injury. It provides a generic or common name for devices. The system allows sharing of data on medical device use, supporting patient safety. GMDN is a global standard, independent.

Global Medical Device Nomenclature Semantic Scholar
August 14, 2023 - The AccessGUDID Database has been updated to include the field for Global Medical Device Nomenclature (GMDN) Code along with the status of the GMDN Code, Active or Obsolete.

(PDF) THE GLOBAL MEDICAL DEVICE NOMENCLATURE DOKUMEN.TIPS
The 12 categories in the GMDN (Global Medical Device Nomenclature) Code table are: Code Term. 01 Active implantable devices. 02 Anaesthetic and respiratory devices. 03 Dental devices. 04 Electro-mechanical devices. 05 Hospital hardware.
