
Converting Dalton to GramMole A Simple Guide
State Dalton's law of partial pressures and use it in calculations involving gaseous mixtures. The molar mass of molecular nitrogen, N 2, is 28.01 g/mol. Substituting this value along with standard temperature and pressure into the gas. (g), measured under the same conditions, was required to prepare this amount of ammonia by reaction.
Dalton’s Atomic Theory The Science Thinkers
As nikie has pointed out, Mathematica considers the Dalton to be a unit of mass. It is also synonymous with the unified atomic mass unit (u).. UnitConvert[Quantity["Daltons"], "u"] 1.0000000u. Mathematica also distinguishes between Molecular Mass and Molar Mass and the default unit for MolecularMass appears to be unified atomic mass unit (u) rather than the Dalton

Dalton's Atomic Theory Graphic Education
The following equation allows you to find the molarity of a solution: molarity = concentration / molar mass. The concentration denotes the mass concentration of the solution, expressed in units of density (usually g/l or g/ml).. Molar mass is the mass of 1 mole of the solute. It is expressed in grams per mole.

Moles To Atoms Conversion Chemistry YouTube
The Molar Volume of a Gas. You will recall that the molar mass of a pure substance is the mass of 6.02 x 10 23 (Avogadro's number) of particles or molecular units of that substance.Molar masses are commonly expressed in units of grams per mole (g mol -1) and are often referred to as molecular weights. As was explained in the preceding lesson, equal volumes of gases, measured at the same.

PPT Dalton’s Atomic Theory PowerPoint Presentation, free download ID6098418
Why is the unit Dalton used instead of just g/mol. Is it to save the key-strokes of writing g/mol every time or is there some more significant reason? I realize Da and g/mol are now not technically the same since the mol was redefined last year, but I don't regularly see protein sizes reported to nine significant figures.

How To Convert Moles To Grams Youtube F3C
The Molar Volume of a Gas. You will recall that the molar mass of a pure substance is the mass of 6.02 x 10 23 (Avogadro's number) of particles or molecular units of that substance.Molar masses are commonly expressed in units of grams per mole (g mol -1) and are often referred to as molecular weights. Equal volumes of gases, measured at the same temperature and pressure, contain equal.

Diagramme de conversion de la mole Chemistry education, Teaching chemistry, Chemistry lessons
In other words, the ratio of amu/atom is the same as the ratio of g/mol.. In other words, at the atomic level, the appropriate unit for amount-specific mass ("molar" mass) is dalton per entity--and, because of the mole definition as an Avogadro number of entities, dalton per entity is exactly equal to the macroscopic units gram per mole or.

Dalton's Atomic Theory — Overview & Modern Application Expii
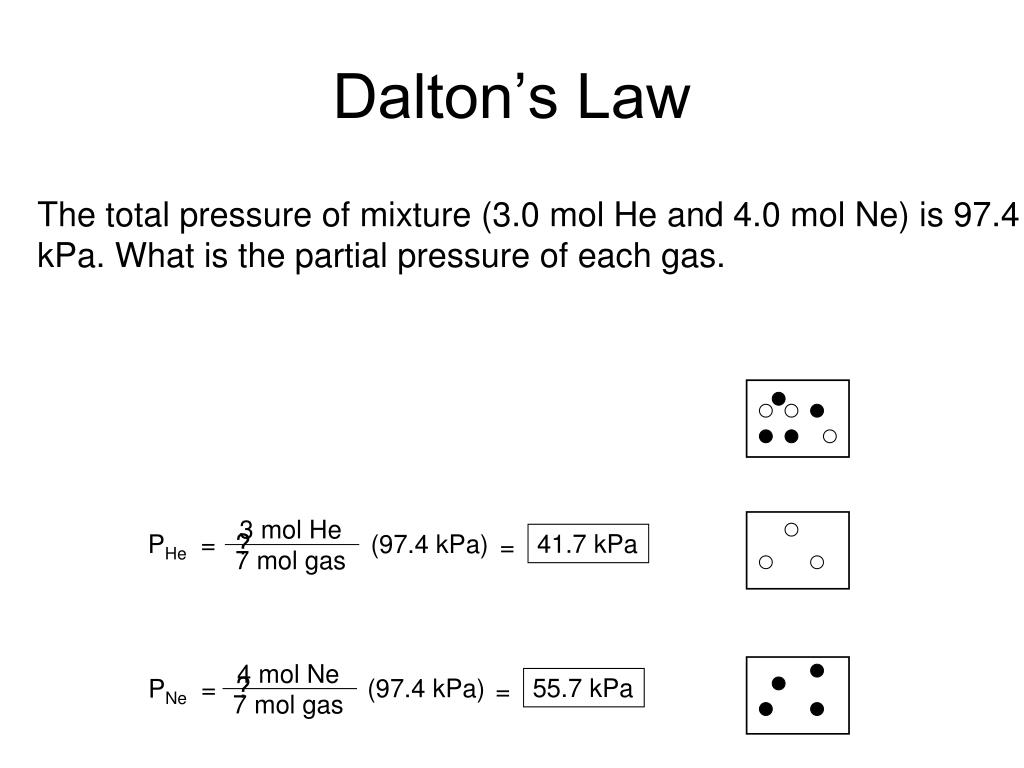
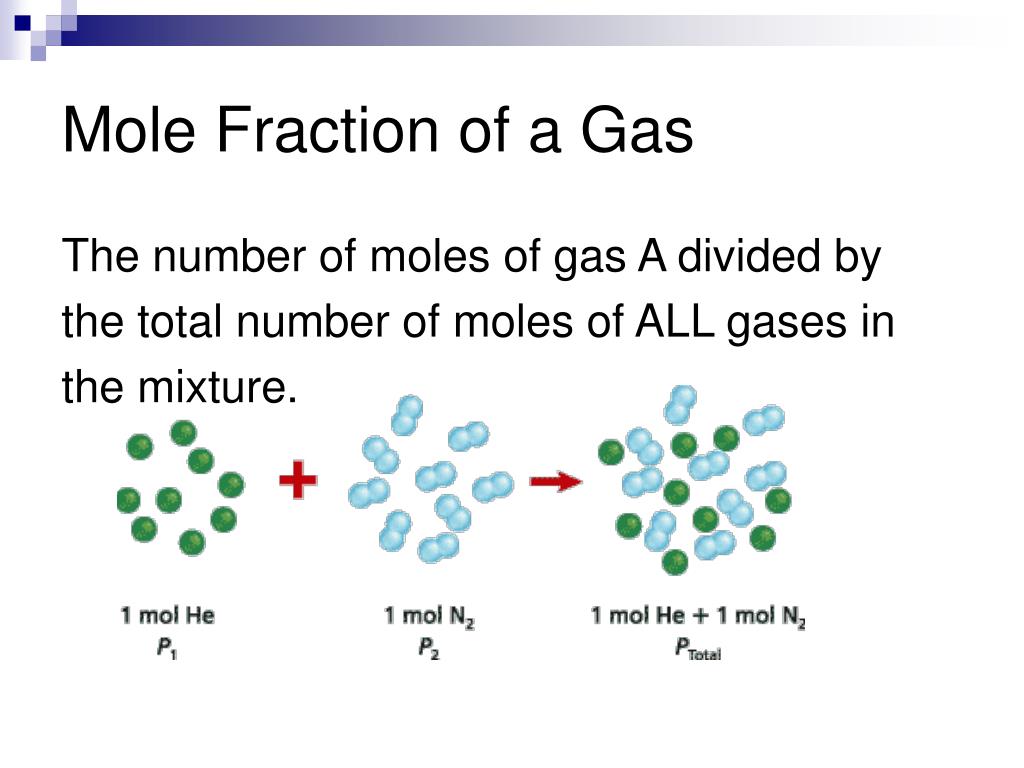
Dalton's law of partial pressures. Dalton's law of partial pressures states that the total pressure of a mixture of gases is the sum of the partial pressures of its components: P Total = P gas 1 + P gas 2 + P gas 3.. where the partial pressure of each gas is the pressure that the gas would exert if it was the only gas in the container.

Converting mole to mass (in grams) YouTube
A dalton (Da) is an atomic mass unit and is equal to 1/12th of the mass of a single atom of Carbon-12. The unified atomic mass unit (u or amu) is equivalent to one twelfth of the mass of one atom of carbon-12. To put it simply, 1 dalton = 1.66053000000133E-24 gram [g]. Grams per mole (g/mol) is a unit that measures the molar mass, whch is.
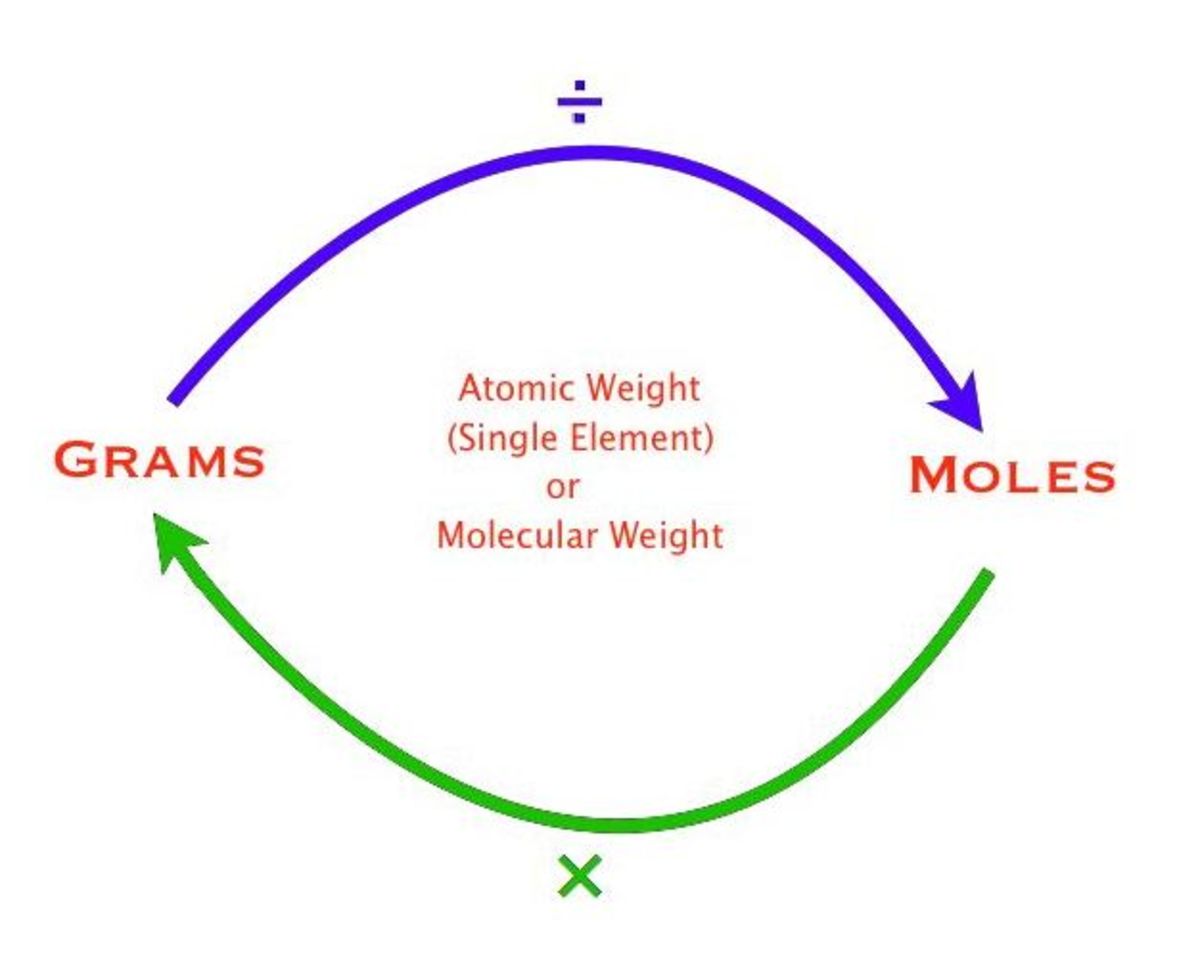
Converting Among Grams, Moles, and Number The Mole Road HubPages
For the most part, you are right. There is a complication though, when converting masses obtained from mass spectrometry (which is the usual context of the unit Dalton) to molar mass, and that is isotopic abundance. For a few elements, there is only one naturally ocurring isotope, but most are a mixture of isotopes with different weights.

Dalton définition et explications
1 dalton = 1.6605300000013E-24 g 1 g = 6.02217364335E+23 dalton. Example: convert 15 dalton to g: 15 dalton = 15 × 1.6605300000013E-24 g = 2.490795000002E-23 g. Popular Weight And Mass Unit Conversions. kg to lbs. lbs to kg. grams to ounces. ounces to grams. pounds to ounces. ounces to pounds. grams to pounds. pounds to grams.

Chemistry Conversion Chart Moles
Molecular Mass (in Daltons): This represents the molecular mass in the Dalton unit (Da), which is a smaller and more precise unit used in the field of molecular biology and biochemistry. Molecular Mass (in g/mol): This denotes the molecular mass in grams per mole (g/mol), which is more commonly used in chemistry and chemical engineering.
PPT Properties of Gases PowerPoint Presentation, free download ID6015649
Convert pressure to same units so 780 torr=1.03 atm; Subtract water vapor pressure from total pressure to get partial pressure of gas A: P A =1.03 atm- 1 atm=0.03 atm; 2. The law of partial pressures also applies to the total number of moles if the other values are constant, so. 4 mol Hydrogen+8 mol Oxygen+12 mol Helium+6 mol Nitrogen=30 moles.
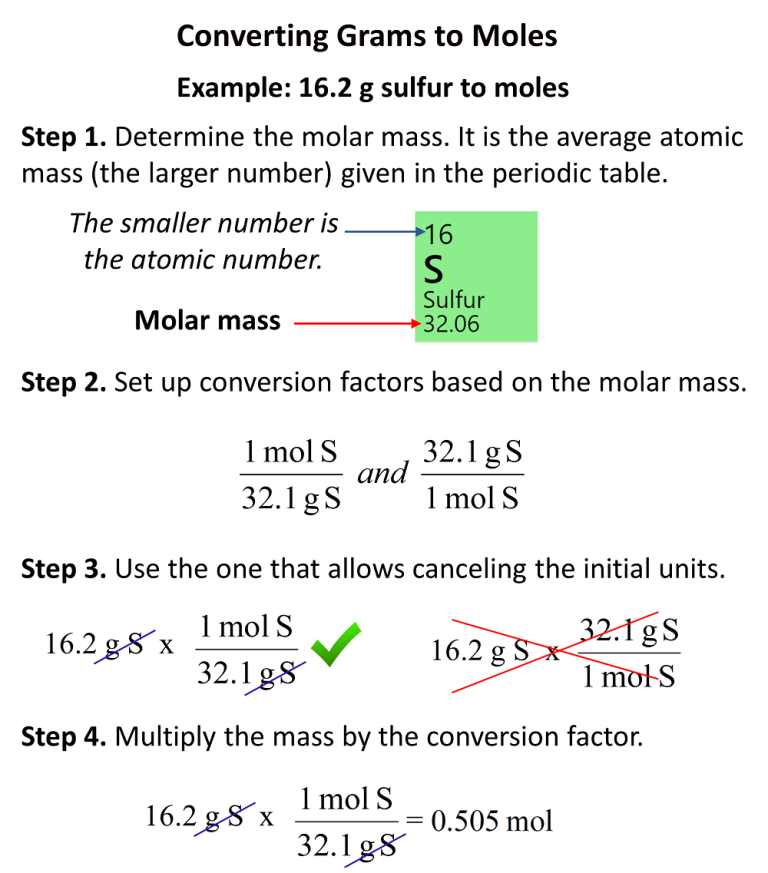
How To Convert Grams To Moles Chemistry Steps
As we just discussed, molar mass is defined as the mass (in grams) of 1 mole of substance (or Avogadro's number of molecules or formula units). The simplest type of manipulation using molar mass as a conversion factor is a mole-gram conversion (or its reverse, a gram-mole conversion).. We also established that 1 mol of Al has a mass of 26.98 g (Example \(\PageIndex{1}\)).

Dalton's Law of Partial Pressure Definition and Examples
The unit of weight is the dalton, one-twelfth the weight of an atom of C-12.. Then you would have the same concentration of molecules in each; that is, drop for drop, each solution would contain the same number of molecules.. Thus 1 mole of glucose weighs 180 g. Furthermore, if you dissolve 1 mole of a substance in enough water to make 1.
PPT Combined Gas Laws and Dalton’s Law of Partial Pressures PowerPoint Presentation ID4146302
For this reason, the dalton (Da) is increasingly recommended as the accurate mass unit. Neither u nor Da are SI units, but both are recognized by the SI. Molar Mass.. When using the unit g/mol, the numerical value of the molar mass of a molecule is the same as its average mass in daltons: Average mass of C: 12.011 Da; Molar mass of C: 12.011.
- Is Breathing Through Your Mouth Unhealthy
- Wat Kost Petroleum In Nederland
- Hoeveel Inwoners Heeft Parijs Nu
- Ronde Van Algarve 2024 Parcours
- Setlist Bruce Springsteen Landgraaf 2023
- Npo Radio 5 Live Luisteren
- Loco Loco Alessio Lyrics Tekst
- Kinderen Van Paul Van Vliet
- Een Foto Zegt Meer Dan Duizend Woorden
- Best Castles In The Netherlands
