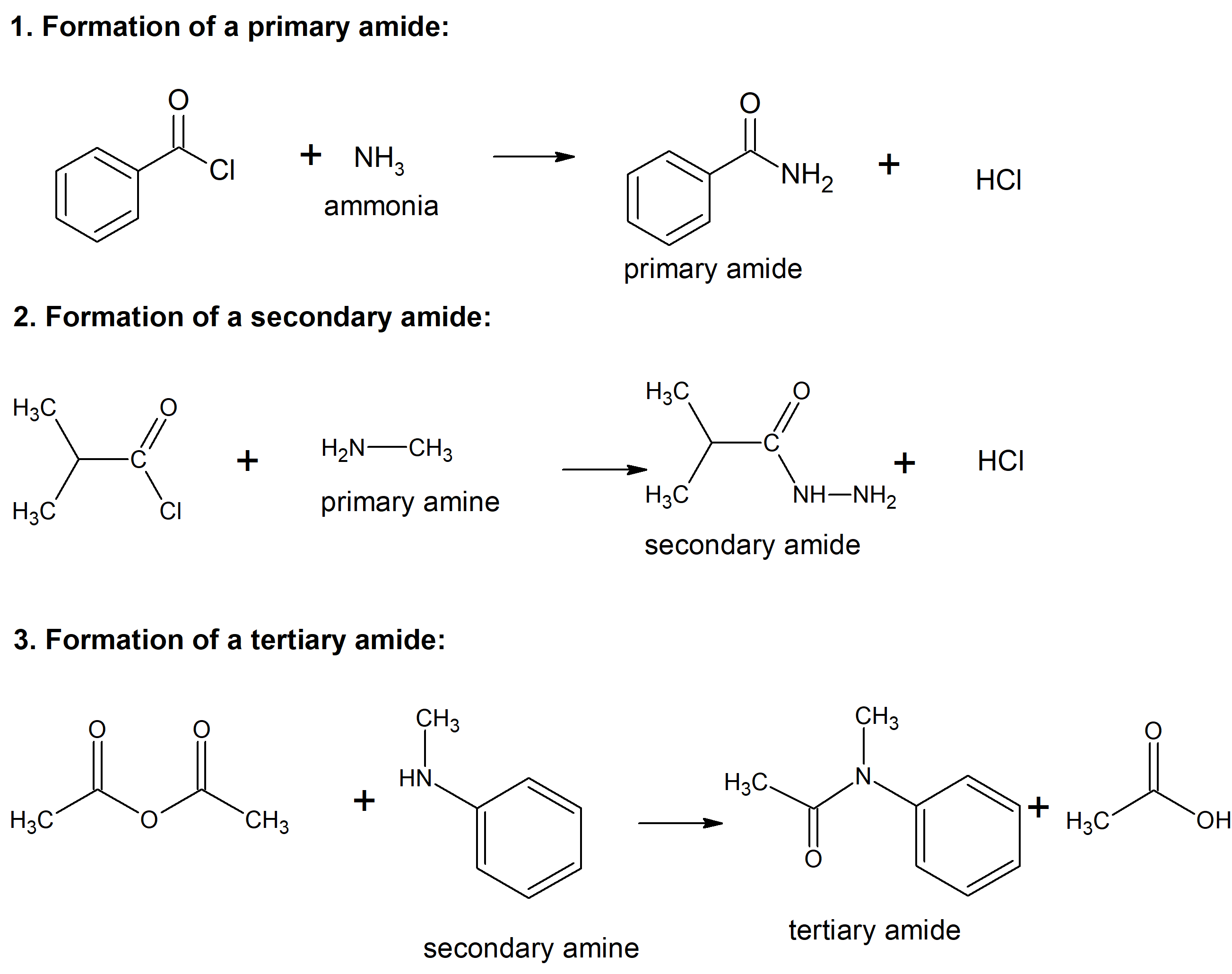
3.9 Chemistry of Amides Synthesis and Reactions Chemistry LibreTexts
Hofmann Elimination Reaction. Like alcohols, amines can be converted into alkenes by an elimination reaction. But because an amide ion, NH 2 -, is such a poor leaving group, it must first be converted into a better leaving group.In the Hofmann elimination reaction, an amine is completely methylated by reaction with an excess amount of iodomethane to produce the corresponding quaternary.

Alcohol Reactions [Reaction Map PDF] Master Organic Chemistry Organic chemistry, Organic
reaction (typically around 200 °C), we have previously reported that the ROP of benzoxazine vitrimers can occur at temperatures as low as 120 °C under catalyst-free con-ditions.39−41 Alcohol groups are known to facilitate the ring-opening reaction through protonation of the oxazine ring initiating subsequent polymerization reactions.42 Endo.
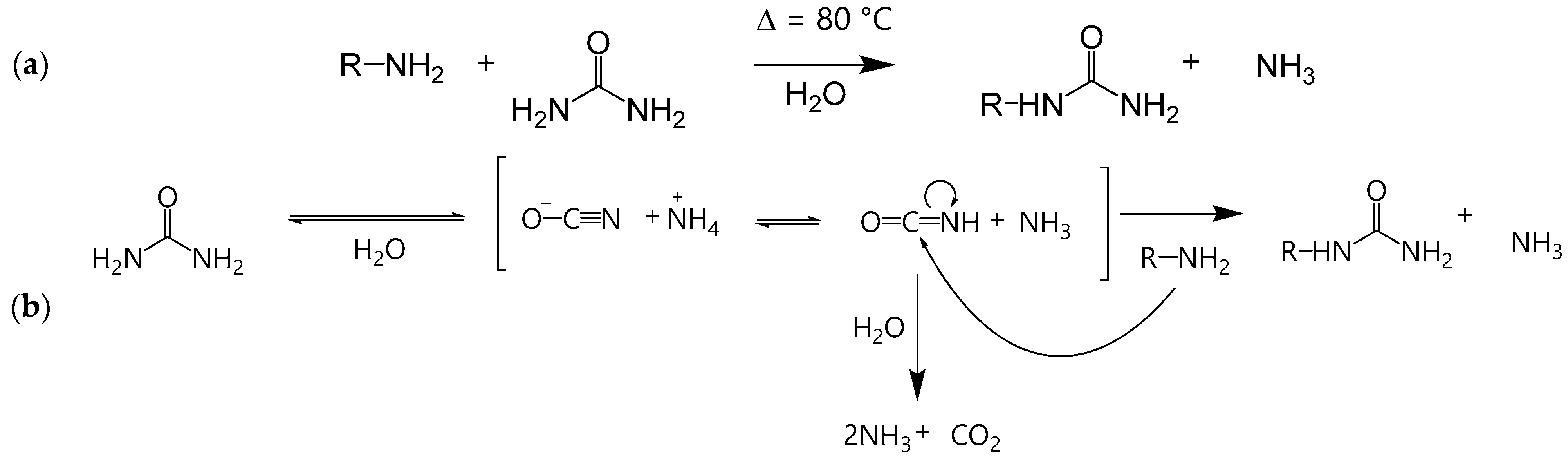
Polymers Free FullText Ureido Functionalization through AmineUrea Transamidation under
27. In the conversion of primary alcohols to primary amines. R − CHX2OH R − CHX2NHX2. direct alkylation of ammonia normally is the last thing you want to do in the lab. Under conditions where OH is a good leaving group, i.e. in acidic medium, the nucleophilicity of ammonia is reduced due to protonation. Moreover, there's little chance to.

The Reaction of Amines with Nitrous Acid Chemistry Steps
The amidation reactions are sensitive to steric hindrance at the α positions of either the alcohol or the amine. Thus, when 2-methyl-1-butanol reacted with benzylamine, the corresponding amide was obtained in 70% yield, with the rest of the alcohol being converted to the ester 2-methylbutyl 2-methylbutanoate (Table 1, entry 4).A similar pattern was also observed when 2-methylhexylamine.

Amine and Alcohol Reaction WillienSendovel
Examples of these kinds of nucleophilic substitutions are the reactions of alcohols, thiols, and amines with alkyl halides to give the corresponding ethers, sulfides, and (secondary, tertiary or quaternary) amines.. The intermediate R-O-BH 3 complex is destroyed by adding aqueous acid to give the final alcohol product. Reactions where.

K2Cr2O7 Reaction With Primary Alcohol Primary Alcohol Substitution Reaction Resulting in Alkyl
One of the most important reasons for using tosylates in S N 2 reactions is stereochemical. The S N 2 reaction of an alcohol with an alkyl halide proceeds with two inversions of configuration—one to make the halide from the alcohol and one to substitute the halide—and yields a product with the same stereochemistry as the starting alcohol. The S N 2 reaction of an alcohol with a tosylate.
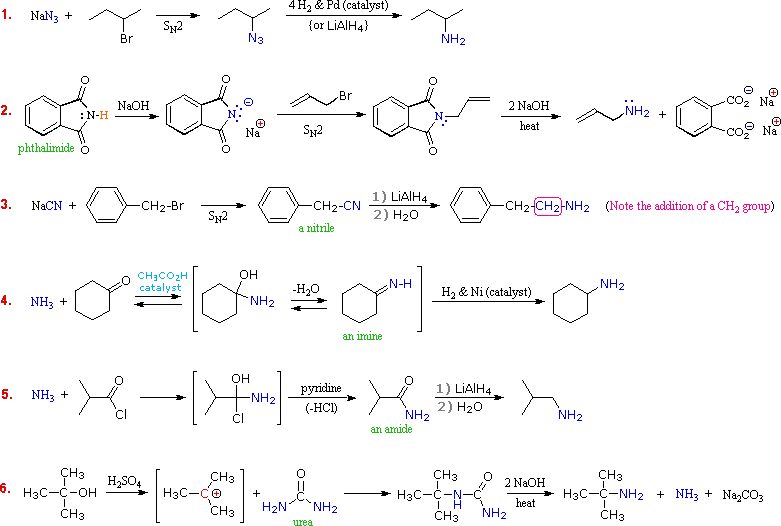
Amine Reactivity
Catalytic N-alkylation of amines by alcohols to produce desired amines is an important catalytic reaction in industry. Various noble-metal-based homogeneous and heterogeneous catalysts have been reported for this process. The development of cheap non-noble-metal heterogeneous catalysts for the N-alkylation reaction would be highly desirable. Hereby, we propose the N-alkylation of amines by.
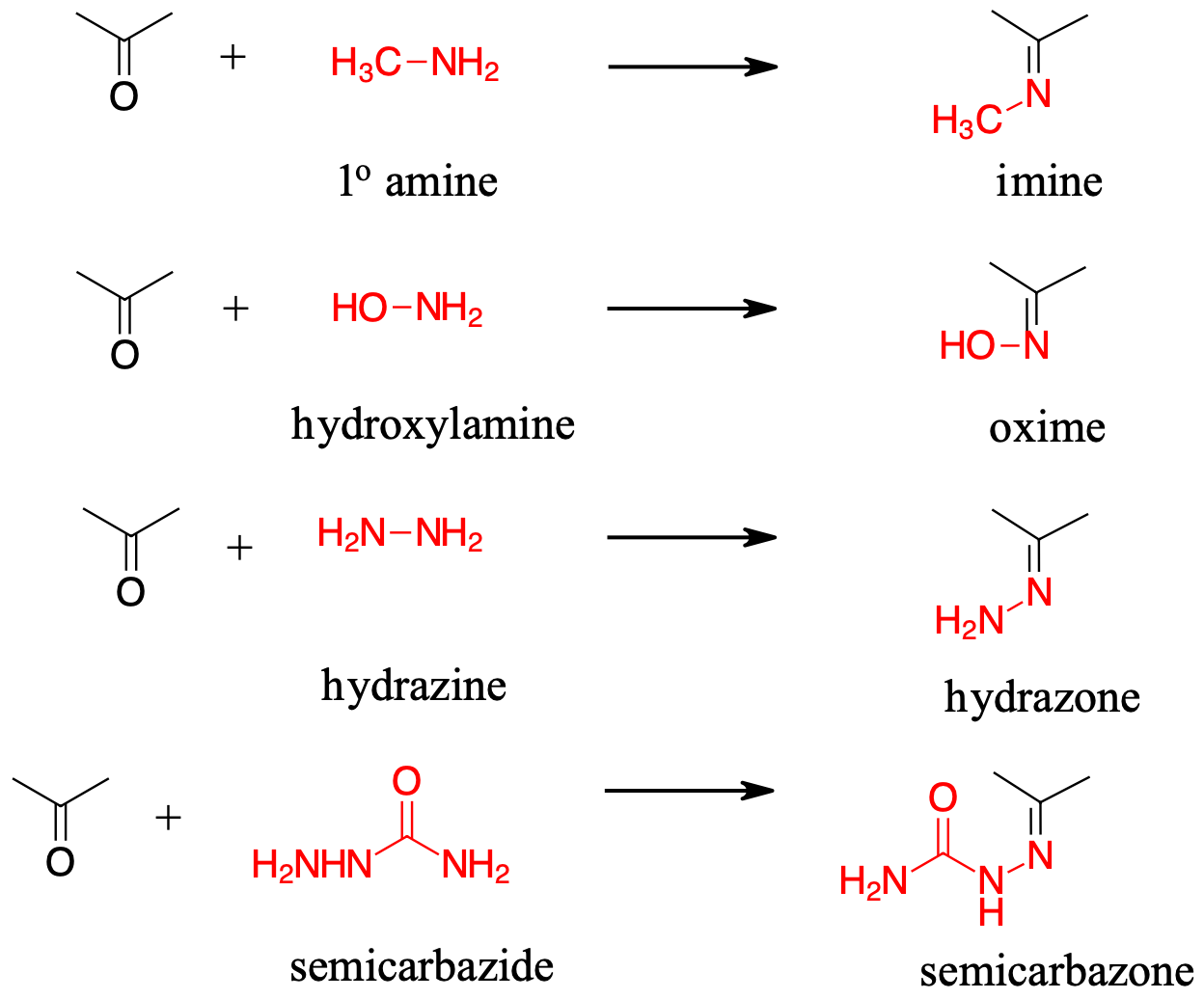
18.8 The Reactions of Aldehydes and Ketones with Amines and Amine Derivatives Chemistry
The N-alkylation of primary amines (1) with alcohols (ROH) is a highly efficient method for preparing secondary alkylamines (2) and tertiary alkylamines (3, Fig. 1b) 4,5,6,7,8,9,10.The most.

Secondary and tertiary amine reactions. MCAT Organic chemistry, Organic chemistry mechanisms
Abstract. We demonstrated in this work the use of affinity ionic liquids, AIL 1 and AIL 2, for chemoselective detection of amine and alcohol gases on a quartz crystal microbalance (QCM). These detections of gaseous amines and alcohols were achieved by nucleophilic aromatic substitution reactions with the electrophilic 1,3,5-triazine-based AIL 1.

What Type Of Product Is Formed In This Reaction Alcohol Amine Ester Images and Photos finder
Pioneering reports dealing with the N-alkylation of amines by alcohols in the presence of homogeneous catalysts were described independently by Watanabe 14 and Grigg 15 at the beginning of the.

Question Video Determining the Structure of the Reaction of an Amine with an Ester Given Their
Various metal and ligand systems have been reported to operate via the BH methodology to carry out many important catalytic transformations 1a one example being the N-alkylation of amines with alcohols. N-substituted amines play an important role in the production of bulk and fine chemicals such as pharmaceuticals, polymers, agrochemicals etc. 2 Traditionally, this transformation has been.
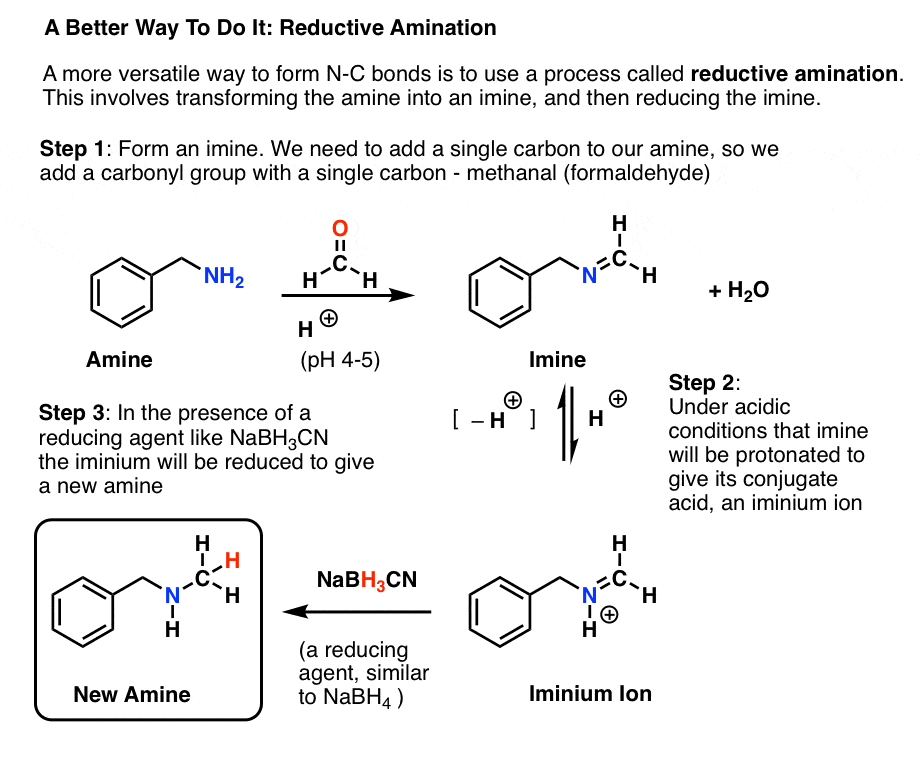
Reductive Amination, and How It Works Master Organic Chemistry
in the reaction between benzyl alcohol 5 and aniline 6 to form amine 7 (Table 1). Using 10 mol % of 1 in toluene, with a 2-fold excess of benzyl alcohol, and trimethylamine-N-oxide to activate the catalyst,6,12 conversion to N-benzyl aniline 7 could clearly be observed and assayed by gas chromatography and 1H NMR spectroscopy of the reaction.

Ch22 Amines
The synthesis of amines through N-alkylation is particularly attractive.Herein, a strategy for visible-light-induced N-alkylation of anilines with 4-hydroxybutan-2-one was developed in the presence of NH 4 Br, which avoid the use of metals, bases and ligands. In addition, gram-scale experiments proved that the system has the potential to be scaled.
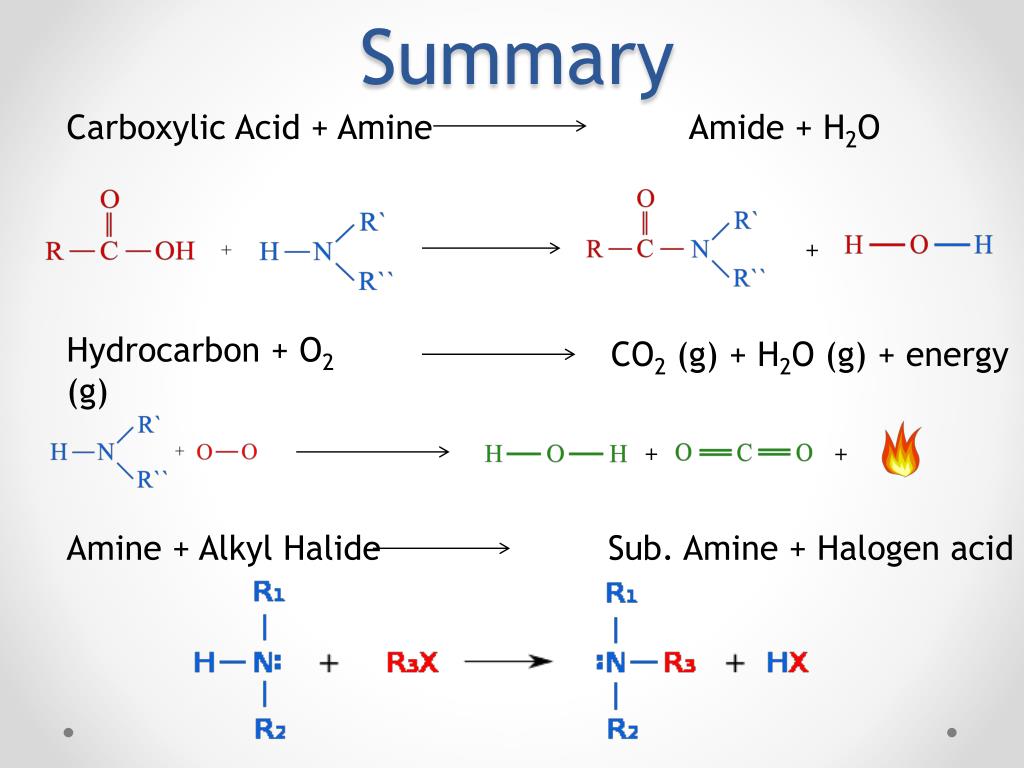
PPT Amine Reactions PowerPoint Presentation, free download ID2118635
Figure 1: Catalytic, direct N-alkylation of amines with alcohols. ( a) Conventional conversion of alcohol into amine via installing a leaving group before nucleophilic substitution with an amine.
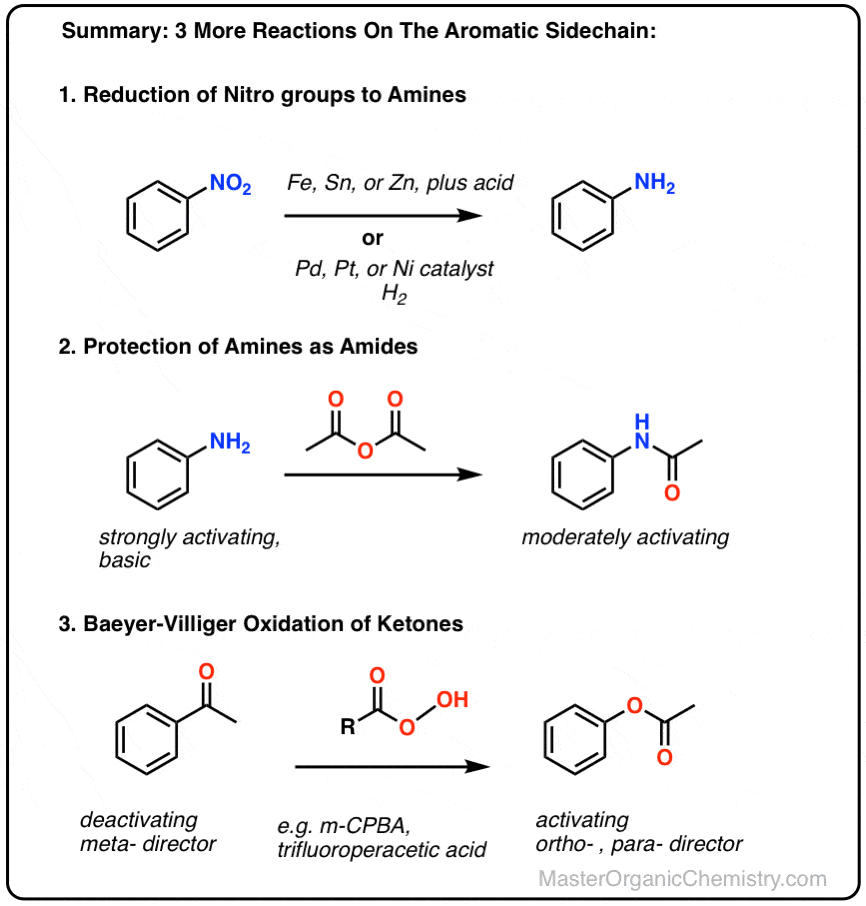
amine and sulfuric acid reaction
If, for example, we wish to carry out an S N 2 reaction of an alcohol with an alkyl halide to produce an ether (the Williamson synthesis), it is necessary to convert the weakly nucleophilic alcohol to its more nucleophilic conjugate base for the reaction to occur. In contrast, amines react with alkyl halides directly to give N-alkylated products.

Reactions of Acid Chlorides (ROCl) with Nucleophiles Chemistry Steps
Developing new click chemistry reactions for robust molecular assembly remains challenging. Here the authors report a light-induced primary amines and o-nitrobenzyl alcohols photoclick cyclization.
